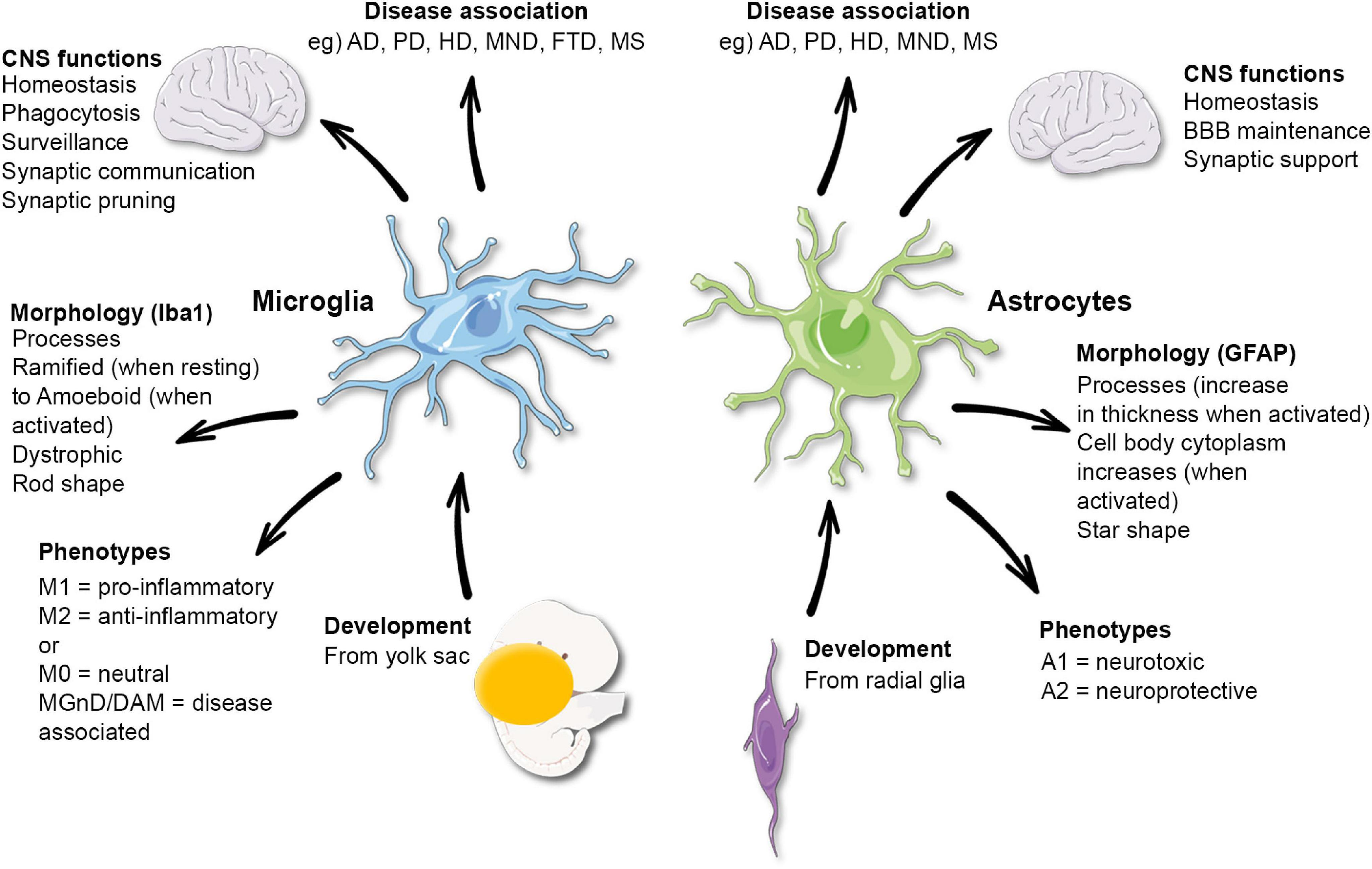
Microglial research has emerged as a pivotal area in understanding the complexities of the brain’s immune system, particularly in relation to Alzheimer’s disease and other neurodegenerative diseases. These unique glial cells are crucial for maintaining brain health by engaging in synaptic pruning – the process of eliminating weak or unnecessary synapses. However, when microglial cells fail to function properly, they can contribute to the progression of conditions like Alzheimer’s, impacting millions of individuals. Leading the charge in this transformative research, Dr. Beth Stevens and her team at Boston Children’s Hospital have illuminated the intricate roles these cells play and their connection to various neurological disorders. This growing field of study not only aims to unravel the mechanisms behind synaptic dysfunction but also promises potential new therapeutic strategies to combat devastating conditions, offering hope for advancements in treatments.
The exploration of microglial cells represents a groundbreaking frontier in neurobiology, often described as the brain’s immune warriors. As researchers delve into the functions of these cells, they uncover their vital role in clearing debris and sculpting neural circuits through a process known as synaptic pruning. Dr. Beth Stevens led crucial investigations into how these immune cells can sometimes misbehave, worsening neurodegenerative diseases such as Alzheimer’s. By revealing the mechanisms behind microglial dysfunction, this research holds the promise of developing innovative biomarkers and treatment options. Ultimately, the continued study of brain immunity will enhance our understanding of mental health and pave the way for breakthroughs not just in Alzheimer’s, but in various cognitive impairments.
The Role of Microglial Research in Neurodegenerative Diseases
Microglial research has emerged as a critical aspect of understanding neurodegenerative diseases, particularly in light of new findings regarding their role in synaptic pruning and brain health. Researchers like Beth Stevens have expanded our knowledge of how these immune cells function within the brain’s immune system, highlighting their dual role for both maintaining and potentially damaging neural networks. In Alzheimer’s disease, for example, microglia are implicated in excessive synaptic pruning, which can disrupt communication between neurons and accelerate cognitive decline.
By investigating the pathways and mechanisms through which microglia operate, scientists are uncovering vital insights that could lead to novel therapeutic targets for conditions like Alzheimer’s and Huntington’s diseases. If we can better understand the triggers that cause microglia to misbehave, researchers can devise strategies to prevent or reverse the synaptic loss and dysfunction associated with these debilitating disorders.
Beth Stevens’ pioneering work in this field underscores the transformative potential of microglial research. Her lab’s findings have paved the way for the development of new biomarkers, which are essential for early diagnosis and may eventually lead to effective treatments. As the connection between the brain’s immune system and neurodegenerative diseases becomes clearer, it offers hope to millions affected by these challenges.
As more researchers join the quest to decode microglial behavior, we can anticipate a richer understanding of how to harness this system for therapeutic benefits. The implications are vast, as they could extend beyond Alzheimer’s disease to a range of neurodegenerative conditions, reinforcing the necessity of continued investment in microglial studies.
Synaptic Pruning and Its Implications in Alzheimer’s Disease
Synaptic pruning, the process by which unnecessary neuronal connections are eliminated, is crucial for healthy brain development and function. However, when this process goes awry, it can contribute to the pathogenesis of neurodegenerative diseases such as Alzheimer’s. Research led by Beth Stevens illustrates how aberrations in synaptic pruning by microglia can lead to greater synaptic loss, a hallmark of Alzheimer’s disease. This mismanagement by the brain’s immune system not only disrupts critical neuronal communication but accelerates the disease process.
Understanding the delicate balance of synaptic pruning has significant implications for treatment approaches in Alzheimer’s disease. By identifying the specific factors that influence microglial behavior, there is the potential for developing interventions to restore normal synaptic pruning mechanisms. As we delve deeper into the complexities of neurodegeneration, it becomes increasingly clear that the kiss of death for neurons might stem from a breakdown in the regulation of synaptic remodeling by microglia.
Moreover, enhancing our knowledge of synaptic pruning opens doors to innovative therapeutic strategies. For example, if we could find ways to inhibit the harmful actions of microglia while promoting their beneficial role, this could drastically change the landscape of neurodegenerative disease management. The reshaping of therapeutic strategies based on synaptic health represents a paradigm shift in how we address diseases like Alzheimer’s.
Thus, ongoing research into synaptic pruning not only aids in understanding disease mechanisms but also highlights potential pathways for new treatments. As the field evolves, the insights gained from understanding microglial functions will be instrumental in combating Alzheimer’s disease and improving patient outcomes.
Advancements in Understanding the Brain’s Immune System
The brain’s immune system, primarily composed of microglia, plays a critical role in maintaining neural health and responding to injury. Recent advancements in research led by scientists like Beth Stevens have shed light on how these immune cells interact with neurons during both normal and pathological states. This newfound understanding signifies a paradigm shift in how scientists approach neurodegenerative diseases, especially conditions such as Alzheimer’s disease.
By unraveling the complexities of microglial functions, researchers are gaining insights into the brain’s immune response and its implications for neurodegeneration. Stevens’ work illustrates how microglia not only protect the brain by clearing debris but can also, under certain conditions, contribute to neuronal damage. This dual nature of microglia is crucial for developing targeted therapies that can restore balance in brain immune functions.
Furthermore, advancements in imaging technologies and molecular biology techniques have facilitated the exploration of the brain’s immune system at unprecedented levels. By visualizing microglial activity and their interactions with neurons in real time, researchers can begin to delineate the mechanisms underlying diseases like Alzheimer’s. This level of detail assists in the identification of novel biomarkers and therapeutic targets that can revolutionize our approach to treating neurodegenerative diseases.
As we continue to explore the intricacies of the brain’s immune system, the potential for new strategies to mitigate the impact of Alzheimer’s and similar diseases becomes clearer. Ultimately, understanding microglial functions will be key in developing interventions that not only slow disease progression but also enhance the overall resilience of the brain.
The Importance of Federal Funding in Neurological Research
Federal funding plays a pivotal role in advancing neurological research, providing essential resources for scientists investigating complex diseases like Alzheimer’s. Beth Stevens’ work exemplifies how vital support from organizations like the National Institutes of Health (NIH) has fueled research that delves into the roles of microglia in neurodegenerative processes. This funding has enabled researchers to pursue innovative avenues, such as exploring the immune functions of microglia and their implications for conditions like Alzheimer’s disease.
Without adequate funding, the cutting-edge studies that lead to groundbreaking discoveries in neurobiology would struggle to progress. The NIH and other federal agencies not only provide financial support but also bolster collaboration among researchers, fostering an environment that encourages sharing of ideas and data. This collaborative spirit enhances scientific inquiry and accelerates the translation of fundamental research into clinical applications.
Moreover, federal backing is crucial for training the next generation of scientists who will advance our understanding of the brain. Programs funded by the government not only support research but also emphasize education and training, ensuring that the field continues to grow. Programs that focus on microglial research and neurodegenerative diseases are especially important, as they help prepare new researchers to tackle the challenges posed by diseases like Alzheimer’s.
As the need for effective treatments grows alongside an aging population, ongoing support for neurological research is imperative. The commitment to investing in this area will facilitate significant advancements that bring us closer to developing therapies that can alter the course of neurodegenerative diseases and improve the lives of millions affected.
Curiosity-Driven Science: A Path to Medical Breakthroughs
Curiosity-driven science has the potential to unlock significant advancements in medical research, particularly in understanding complex diseases. Beth Stevens’ journey emphasizes how foundational research—driven by sheer curiosity rather than immediate application—can lead to unforeseen medical breakthroughs. This approach has allowed her to explore the intricacies of the brain’s immune system and its role in diseases such as Alzheimer’s, ultimately reshaping the landscape of neurological research.
When scientists follow their curiosity, unexpected discoveries can lead to transformative changes in our understanding of diseases. Stevens’ hunch about the role of microglia in synaptic pruning demonstrates how basic science can pave the way for applied research that directly impacts patient care. This highlights the importance of supporting scientific inquiry without the constraints of immediate expectations, as it often leads to groundbreaking insights that change the course of treatment for conditions like Alzheimer’s.
Additionally, the investment in curiosity-driven projects serves to motivate researchers to explore bold hypotheses that may otherwise be dismissed in traditional research frameworks. The ability to ask ‘what if’ and follow the resulting leads can produce innovative solutions to longstanding medical challenges. This mindset fosters a culture of creativity and exploration within scientific disciplines.
In conclusion, curiosity-driven research is essential for the evolution of medical science. It encourages researchers to forge new paths and yields insights that can ultimately lead to the development of more effective treatments for neurodegenerative diseases, ensuring that we are not simply addressing the symptoms but also understanding the root causes.
Synapse Sculpting: The Essential Role of Microglia
Synapse sculpting, a dynamic process mediated by microglial activity, is fundamental for maintaining optimal brain function. This intricate balance of formation and elimination of synapses ensures that neural circuits function correctly. However, when microglial action becomes excessive, as observed in conditions like Alzheimer’s disease, it may lead to detrimental loss of synaptic connections, further exacerbating neurodegeneration.
Beth Stevens’ research highlights the importance of microglia in this process, establishing critical links between synapse health and cognitive function. The Stevens Lab’s findings showcase how microglial dysfunction can disturb the delicate equilibrium needed for healthy brain signaling. This understanding opens up new avenues for therapeutic interventions aimed at correcting the synaptic pruning process gone awry, a step crucial for combating Alzheimer’s and related diseases.
Furthermore, the implications of microglial role in synaptic sculpting extend beyond just neural communication; they relate to overall brain health. Studies on how microglia interact with synapses provide insights into how these immune cells can influence neuroplasticity and learning. Thus, a deeper grasp of microglial behaviors offers opportunities to enhance cognitive health in aging populations.
In summary, the role of microglia in synaptic sculpting is a double-edged sword, with the potential to either protect or harm neuronal networks. By refining our understanding and addressing microglial dysfunction, researchers can work towards therapeutic solutions that not only prevent neurodegeneration but also enhance lifelong cognitive function.
Exploring the Future of Neurodegenerative Disease Research
The future of neurodegenerative disease research promises exciting advancements as scientists delve deeper into the complexities of conditions like Alzheimer’s disease. With significant progress in understanding the roles of microglial cells, particularly their impact on synaptic pruning, the foundation is being laid for innovative treatment strategies. Beth Stevens and her counterparts are at the forefront of this exploration, investigating the interplay between immune responses and neurodegeneration.
Emerging technologies, including advanced imaging and genetic manipulation techniques, are enhancing researchers’ ability to track microglial dynamics in live subjects. These innovations will likely uncover new facets of how neurodegenerative diseases progress, allowing for earlier intervention strategies. Furthermore, as we uncover the molecular underpinnings of microglial behavior, we can better tailor therapies that mitigate their adverse effects while enhancing their protective roles.
As research continues to evolve, researchers are also gaining an appreciation for the environmental and genetic factors that may influence microglial function. Such insights can lead to the identification of risk factors connected to neurodegenerative disease, moving the field toward more personalized medicine approaches.
In conclusion, the future of neurodegenerative disease research, bolstered by a focus on microglial activities, is bound to yield significant breakthroughs that enhance understanding and treatment of Alzheimer’s and other conditions. Continued investment in this area is crucial, as it holds the key to unlocking therapeutic avenues previously unimagined.
Understanding Alzheimer’s Disease Through the Lens of Basic Science
Basic science serves as the cornerstone of our understanding of complex conditions like Alzheimer’s disease. By focusing on fundamental biological processes, researchers like Beth Stevens can investigate the mechanisms that drive neurodegeneration. Her work on microglial activity exemplifies the necessity of foundational research in illuminating the pathways that lead to challenges in brain health. Such studies provide a framework for exploring how these immune cells influence synaptic health and overall neural function.
As researchers unravel the basic processes involved in Alzheimer’s, they can begin to stitch together a tapestry of knowledge that informs treatment development. Each discovery builds upon the last, creating a comprehensive picture that guides therapeutic strategies. By prioritizing curiosity-driven exploration, scientists can uncover links between microglial dysfunction and cognitive decline, enabling new insights into potential interventions.
Moreover, understanding Alzheimer’s through the lens of basic science reveals possibilities for prevention and treatment strategies that may alter disease trajectories. Knowing how microglia impact synaptic pruning can inform the development of therapies aimed at modulating their function to promote better outcomes for patients.
Ultimately, the synergy between basic science and clinical application paves the way for a future where Alzheimer’s disease may not just be managed but potentially prevented or reversed. This interconnected approach will be essential as we strive to combat the Alzheimer’s epidemic and improve the lives of millions.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s disease?
Microglial cells act as the brain’s immune system, playing a critical role in regulating synaptic pruning and clearing out dead or damaged cells. In Alzheimer’s disease, abnormalities in microglial activity can lead to excessive pruning of synapses, contributing to neurodegeneration and cognitive decline.
How does microglial research contribute to the understanding of neurodegenerative diseases?
Microglial research sheds light on the mechanisms by which these immune cells respond to neuronal health. By studying microglia, scientists like Beth Stevens have uncovered how their dysfunction can lead to synaptic loss and the progression of neurodegenerative diseases, including Alzheimer’s and Huntington’s.
What is synaptic pruning, and how is it related to microglial function?
Synaptic pruning is the process by which the brain eliminates excess synapses to enhance neural efficiency during development and in response to injury. Microglial cells are pivotal in this process, and their dysregulation can lead to conditions like Alzheimer’s disease, as they may prune synapses too aggressively.
What discoveries have been made in microglial research by Beth Stevens?
Beth Stevens, through her research at Boston Children’s Hospital, has uncovered that aberrant microglial activity can significantly influence the development of Alzheimer’s disease and other neurodegenerative disorders. Her findings are paving the way for new biomarkers and treatment strategies.
Why are microglial cells considered essential for brain health?
Microglial cells are essential for brain health as they monitor the brain environment, respond to injury, and maintain neuronal connections through synaptic pruning. Their proper functioning is crucial to prevent neurodegenerative diseases, including Alzheimer’s.
How does funding support affect microglial research?
Federal funding, such as from the National Institutes of Health, has been instrumental in advancing microglial research. It enables scientists like Beth Stevens to explore fundamental questions about brain immunity and the implications of microglial dysfunction in neurodegenerative diseases.
What advancements in treatment stem from microglial research for Alzheimer’s patients?
Advancements from microglial research, particularly from studies conducted by the Stevens Lab, have the potential to lead to the development of new biomarkers and therapeutic approaches aimed at mitigating the effects of Alzheimer’s disease, providing hope for millions affected.
How do microglia modulate brain circuits during development?
During brain development, microglial cells prune unnecessary synapses to refine brain circuits, ultimately shaping cognitive function. Research indicates that improper microglial activity during this stage can lead to long-term neurodegenerative consequences, such as in Alzheimer’s disease.
| Key Points |
|---|
| Microglial cells are crucial for brain immunity, clearing damaged cells, and pruning synapses. |
| Aberrant microglial pruning is linked to neurodegenerative diseases like Alzheimer’s and Huntington’s. |
| Beth Stevens’ lab combines basic science and curiosity-driven research to advance understanding of microglia. |
| Federal funding, primarily from NIH, has been vital for the foundational research in microglial functions. |
| Research on microglia opens new paths for biomarkers and therapeutic interventions for Alzheimer’s. |
| Stevens emphasizes the importance of basic research in leading to practical medical advancements. |
Summary
Microglial research is at the forefront of understanding brain immunity and its implications for neurodegenerative diseases. As demonstrated by Beth Stevens and her groundbreaking studies, microglia are not only defenders of the brain but also crucial players in synaptic pruning. The insights gained from her collaborative efforts, supported by NIH funding, pave the way for potential new biomarkers and treatments for Alzheimer’s and related disorders. This evolving field underscores the importance of curiosity-driven research, which can lead to significant advancements in medical science and improve the lives of millions.